Submitted on August 2, 2013
UCSF urologists are actively enrolling interested candidates undergoing robot assisted radical prostatectomy (RARP) to a new study that utilizes a patient’s own tissue to create a hammock-like support for the urethra called a “sling.” Investigators are testing this technique, first developed here at UCSF, to see if the sling helps decrease urinary leakage post RARP.
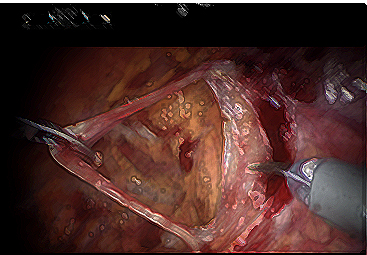
This research is being done because many men after radical prostatectomy are at risk of having urinary leakage, which can affect their quality of life. For those men who have significant leakage after removal of the prostate, a separate procedure performed after RARP, which involves placement of a similar sling made of synthetic material, has shown good results in decreasing the amount of urinary leakage. By creating a hammock like support for the urethra and bladder neck, the sling helps to decrease urinary leakage.
Previous studies have shown favorable results with respect to urinary control when placing a sling at the time of open radical prostatectomy, when the prostate is removed through an open incision from the pubic bone to the belly button. However, this has never been recreated in patients undergoing removal of their prostate with robot assisted laparoscopic surgery. UCSF urologists have published their preliminary results, which suggest the sling may help in improving recovery of urinary control, without causing any adverse effects. However, further testing is required to confirm a benefit of the sling in improving continence. The technique involves use of the vans deferens to act as the sling. The vans deferens is a tube that carries sperm from the testis to the prostate. This tube is no longer needed once the prostate is removed. Skilled surgeons “harvest” the vans deferens and attach to support to the urethra and bladder neck.
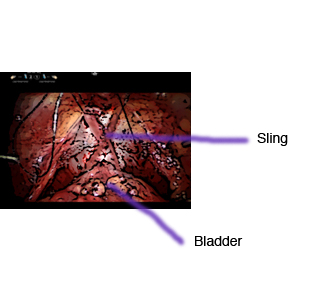
The study, which is a randomized clinical trial among patients undergoing RARP, has two arms: sling/no-sling. Patients who consent for the study have a 50/50 chance of receiving sling in addition to RARP versus RARP alone.
The primary study outcome will be the proportion of patients requiring 0 pads per day 6 months after removal of the Foley catheter post-surgery for the sling and control subsets of patients. This will be determined over the phone by a qualified member of the research personnel blinded to the treatment assignment using scripted questions.
For more information about this study, please contact urology fellow Christopher Welty at 415/885-7298.