Submitted on September 6, 2016
UCSF’s multidisciplinary urology team is expanding treatment options for bladder cancer to include therapy that helps ramp up the immune system. A clinical trial that opened in March is using a type of drug called a checkpoint inhibitor to help the body‘s immune cells fight cancer that is confined to the bladder. In selected patients, it will replace the course of chemotherapy typically given to shrink the tumor before surgical removal of the bladder (cystectomy).
The drug, atezolizumab, has been used with good early results in patients with bladder cancer who fail to respond to chemotherapy, and it received FDA approval for this use in 2016. The new clinical trial is exploring atezolizumab’s efficacy as an alternative in patients who cannot receive chemotherapy because of poor kidney function or other health complications.
“Immunotherapy is rapidly evolving, and it offers promising new treatment options for many cancers. We are excited to offer this trial here at UCSF,” said Peter Carroll MD, MPH, chair of the Department of Urology.
Bladder cancer occurs nearly three times more often in men than women, and is related to increasing age and to environmental factors, particularly smoking.
At UCSF, care of bladder cancer patients is coordinated by a multidisciplinary team that includes urologists, medical oncologists, radiation oncologists and many other health care professionals, including those who focus on symptom management and nutrition.
The immunotherapy trial is open to patients whose cancer has invaded the muscle wall of the bladder but has not metastasized to other organs, and also to patients who have had a recurrence of a superficial cancer confined to the bladder lining. Researchers plan to enroll a total of 42 patients through UCSF and a second arm of the study set to open at USC Medical Center later this spring.
Scientists will examine bladder tissue once the organ is removed to observe how immunotherapy has affected cancer cells and to identify biomarkers that may predict which patients respond.
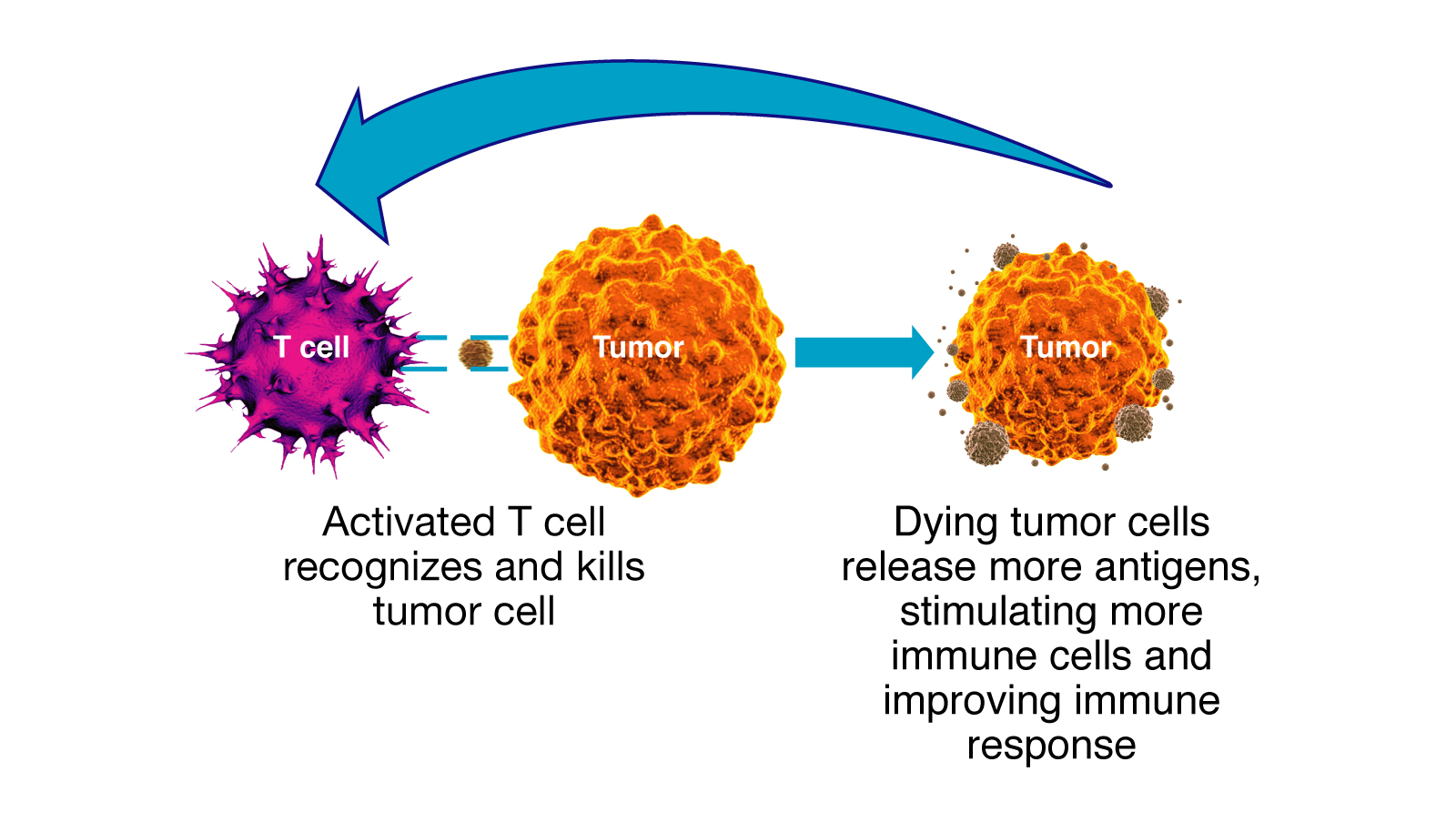
“Our hope is that these checkpoint inhibitors will not only knock out bladder cancer cells before surgery, but also sensitize the immune system so that the body preserves an immunologic memory of the cancer cells and can attack any recurrence early in the process,” said medical oncologist Terence Friedlander, MD. He is part of a multidisciplinary team at the Helen Diller Family Comprehensive Cancer Center working under the direction of Lawrence Fong, MD, on immunotherapy for a variety of cancers.
“This provides another tool in our multimodal strategy for treating invasive bladder cancer,” said urologist Sima Porten, MD, MPH, another member of the multidisciplinary team. “Our ultimate goal will be to determine which sequence of therapies will work best in an individual patient, as we work toward personalized approaches.”
Ramping up the immune response
Checkpoint inhibitors work by interfering with proteins that put the brakes on the immune response. This class of drugs has been used with significant success in patients with metastatic melanoma, lung and kidney cancers, in addition to metastatic bladder cancer. In these settings, about 15-25% of patients have a durable response, in which their tumors shrink and stabilize for a significant period of time. Researchers are now starting to explore whether these agents are effective with nonmetastatic disease.
Atezolizumab targets a protein called PD-L1 on the surface of tumors that halts the immune response by binding with PD-1 on the surface of immune cells. By impairing the binding of these two proteins, atezolizumab allows the immune response to proceed and attack the tumor.